Part 1: Light Hydrocarbon Gases
Why should we care about the solubility of gases in water?
The solubility of hydrocarbons and non-hydrocarbons like carbon dioxide and hydrogen sulfide in water is of interest for oil and gas production and processing facilities dealing with water treatment. It is also important for disposal facilities from an environmental aspect. In addition, determination of the solubility of these components in aqueous phase is critical in the study of the gas hydrates kinetics [1].
In Part 1 of this series, we will focus on the solubility of light hydrocarbon gases such as CH4, C2H6, C3H8, iC4H10, and nC4H10. We will review and present example available experimental solubility data and a thermodynamic model. For the case study, we will present diagrams to show the effect of temperature and pressure on the solubility of these light hydrocarbon gases in water.
Solubility of Light Hydrocarbon Gases
In general, the solubility of a gas (solute) in water (solvent) is a function of temperature, pressure, and composition. The solubility of gases in water can be measured experimentally or estimated by thermodynamic models using equation of state or a correlation based the Henry’s law.
In the thermodynamic model [2] developed by Zirrahi et al., the Peng-Robinson equation of state coupled with a non-random mixing rule is used to model gas phase (applicable to sweet, sour, or acid gases). The aqueous phase is modeled by Henry’s law approach (applicable to the fresh and brine water). They reported good agreement between their model and experimental data available in the literature. The aqueous phase solubility and water content of pure CH4, CO2, and H2S were represented with absolute average relative deviations of less than 6.3% and 5.6%, respectively.
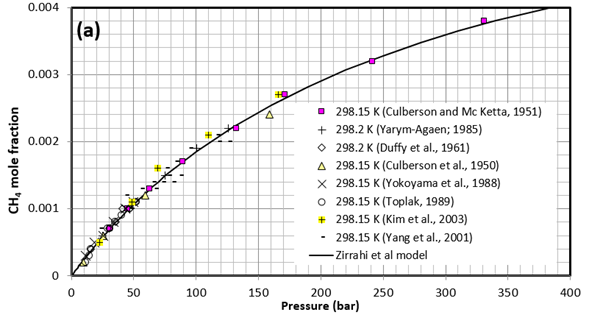
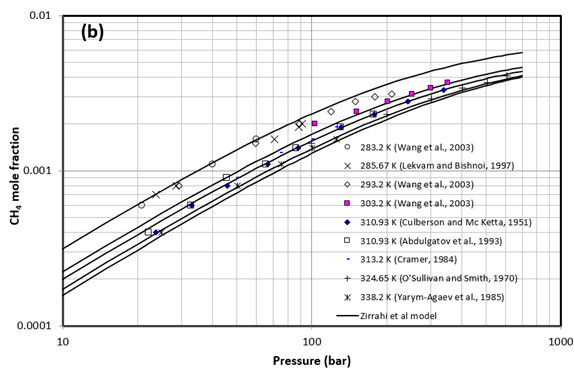
Figure 1 presents a comparison between the experimentally measured CH4 solubility in water by different investigators and the results of the proposed model by Zirrahi et al. [2]. Solid lines are the results obtained from the proposed model at (a) 298.15 K (25°C), and (b) 338.2 K (65.1°C), 324.65 K (51.5°C), 310.93 (37.8°C) K, 303.2 K (30.1°C), and 285.67 K (12.5°C).
Figure 1. Comparison between solubility of CH4 experimental data and results of a proposed model by Zirrahi et al. [2]. Solid lines are the results obtained from the model at (a) 298.15 K (25°C), and (b) 338.2 K (65.1°C), 324.65 K (51.5°C), 310.93 (37.8°C) K, 303.2 K (30.1°C), and 285.67 K (12.5°C).
Case Study
To investigate the effect of temperature, pressure, and composition on the solubility of light hydrocarbon gases in water, we considered a lean gas mixture with the composition shown in Table 1.
Table 1. Composition of gas mixture
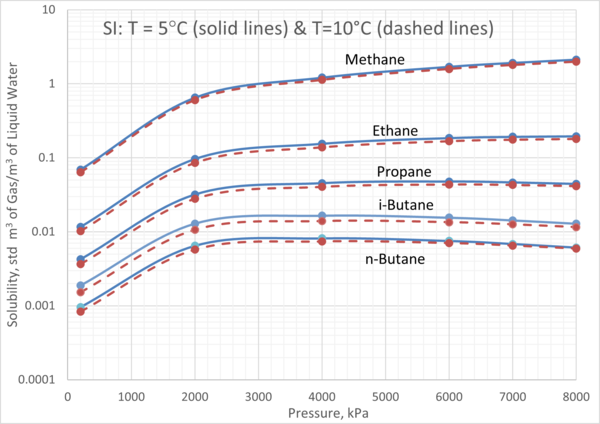
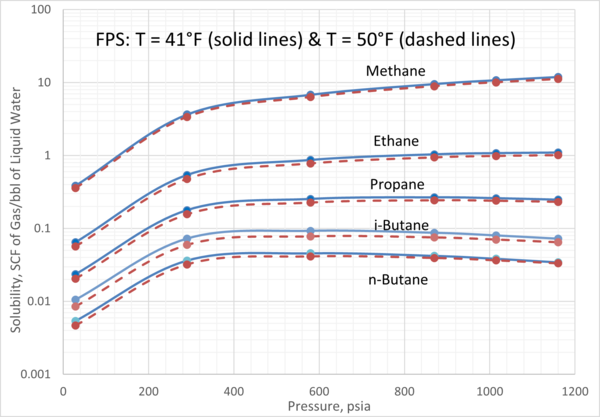
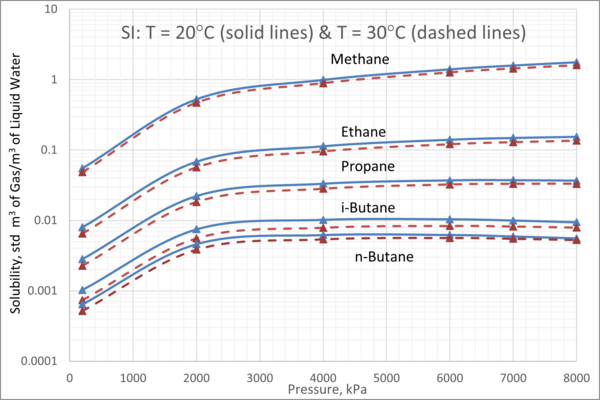
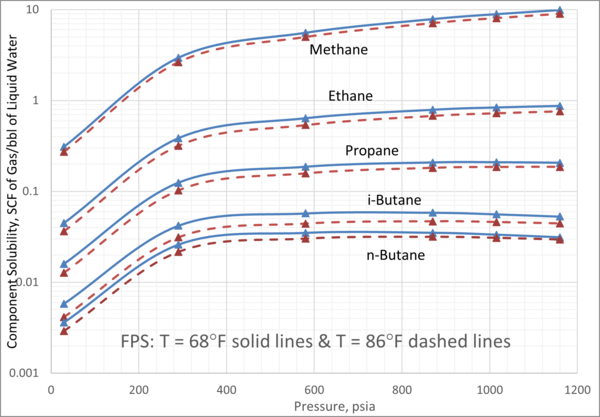
We obtained the solubility of each component in water from ProMax [3] using the Peng-Robinson equation of state. Four isotherms of 5°C, 10°C, 20°C, and 30°C (41°F, 50°F, 68°F, and 86°F) were selected. For each isotherm, the pressure was varied from 200 kPa to 8000 kPa (29 psia to 1160 psia). The calculated gas solubility results, std m3 of gas per m3 of water (SCF of gas per bbl of water) are presented in Figures 2 and 3.
Figures 2 and 3 indicate that:
1. For a given pressure and temperature, methane has the highest and n-butane has the lowest solubility in the aqueous phase.
2. For an isotherm, methane and ethane solubility increase as pressure increases.
3. For an isotherm, propane solubility increases as pressure increase up to 3000 kPa (435 psia) beyond which remains constant.
4. For an isotherm, i-butane and n-butane solubility increase as pressure increases up to 3000 kPa (435 psia) beyond which decreases with pressure increase.
Figure 2. Variation of solubility of CH4, C2H6, C3H8, iC4H10, and nC4H10 with pressure and at low temperatures
Figure 3. Variation of solubility of CH4, C2H6, C3H8, iC4H10, and nC4H10 with pressure and at moderate temperatures
Summary
This TOTM presented the solubility of light hydrocarbon gases, i.e., methane through normal butane, in water as a function of pressure and temperature. Simple charts (Figures 1a and b) to estimate the solubility of pure methane in water were presented. Using ProMax [3], the solubility charts for a mixture of methane through normal butane (Table 1) in water for four isotherms are also presented (Figures 2 and 3).
To learn more about similar cases and how to minimize operational problems, we suggest attending our G4 (Gas Conditioning and Processing), G5 (Practical Computer Simulation, and Applications in Gas Processing) courses.
By: Mahmood Moshfeghian, Ph.D.
References:
- Sloan, E.D., Koh, C.A., Clathrate Hydrates of Natural Gases, 3rd ed., CRC Press, 2008.
- Zirrahi, M., Azin, R., Hassanzadeh, H., Moshfeghian, M. “Mutual solubility of CH4, CO2, H2S, and their mixtures in brine under subsurface disposal conditions,” Fluid Phase Equilibria, Fluid Phase Equilibria 324, 80–93. 2012
- ProMax 5.0, Build 5.0.20034.0, Bryan Research and Engineering, Inc., Bryan, Texas, 2020.