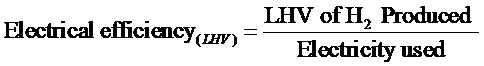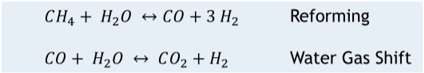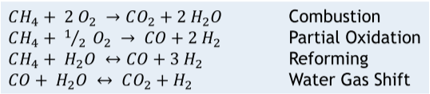Kindra Snow-McGregor, P.E., Ron Frend, Brian Inglis, Karl Gerdes
Introduction…The factors driving the interest in hydrogen....GHG emissions and $$$$
Low or no carbon hydrogen production, distribution and consumption is thought to be one of the primary solutions to reaching net zero for heavy industry, possibly power generation, residential use for heating and cooking, as well as transportation. Hydrogen has a high heating value, and the combustion reaction does not produce CO2. According to H2 Tech, there are currently 876 green hydrogen projects, and 245 blue hydrogen projects ongoing.
An example of the level of attention, in 2021 the U.S. passed the Infrastructure, Investment and Jobs Act (IIJA) which contains $9.5 billion in funding for hydrogen1. $8 billion of that is dedicated to the development of hydrogen hubs (H2Hubs) in the U.S.. In 2022, the Inflation Reduction Act (IRA) contained two tax provisions that will subsidize clean hydrogen production2. The U.S. Department of Energy (DOE) defines H2Hubs as a network of clean hydrogen producers, potential consumers, and connective infrastructure that are all located in close proximity to one another. The DOE will release the funding to six, and possibly up to ten, clean hydrogen hubs around the country.
The interest in clean hydrogen is not just a U.S. trend. The U.K. has developed a Hydrogen Strategy3. Japan4, Australia5 and other countries are also looking to hydrogen as a key part to the solution of combatting climate change. The EU commission also plans on subsidizing “green” hydrogen to the tune of €800 million and will offer a “fixed premium” per kg of “green” hydrogen produced, subsidizing this production over a 10-year period6.
Given the amount of investment and interest in hydrogen, we have decided to publish a series of “Tips of the Month” to explore the opportunities, challenges, and potential solutions to hydrogen applications and uses; this is the first paper in the series. As such, we will start this exploration from the beginning – what are the colors of hydrogen? How are they produced? What are their technical challenges for deployment? After that we will review the USE case for hydrogen. Why is there such an interest and focus for these investments?
Our future tips will explore the possible end uses and benefits of hydrogen, thermodynamics of natural gas use versus hydrogen in industrial applications, safety considerations, transportation challenges and opportunities, storage, and end use capabilities. In addition, we will take a look at some cost comparisons, where possible of the different hydrogen production options. We hope you enjoy taking this journey with us. Our aim is to take a thermodynamically balanced non-biased view of possible applications, costs, and implications.
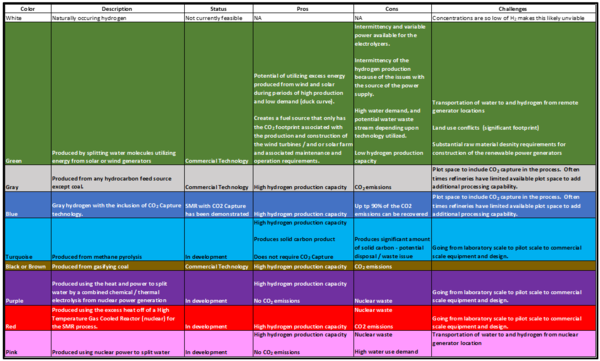
Hydrogen Colors – What do they mean?
“White” Hydrogen”:
Hydrogen as an atom is common in our earthly system bound up as water, hydrocarbons, plants, animals, and of course humans. Hydrogen is the most abundant chemical element in the universe. However, pure hydrogen as a molecule H2, is exceptionally rare in our environment. The hydrogen molecule is the lightest molecule with a molecular weight (MW) of 2, and is the first element in the periodic table. Naturally occurring hydrogen is referred to as White Hydrogen. Given the scarcity of naturally occurring white hydrogen it is not currently feasible to recover and concentrate white hydrogen for large scale use and deployment.
“Green” Hydrogen”:
Green hydrogen is produced through water electrolysis powered by renewable (wind or solar) energy installations. It should be noted, for hydrogen to be truly “green” that it must only be produced utilizing renewable energy. There are no “net-zero” grids anywhere in the world today. Thus, if the electrolyzers are supplemented with grid power for the times when the solar or wind plant are not producing sufficient electrons to run the unit, the hydrogen then has the CO2 footprint of whatever grid CO2 intensity has that it is supplied with. Coal and natural gas are the most common power generators providers globally. The technology to produce hydrogen via electrolysis is well known and has been deployed at a large scale since the 1920’s, although typically for small specialty applications. The new focus is how to upscale electrolysis coupled with renewable power generation to produce hydrogen at a large scale.
“Gray Hydrogen”:
Gray hydrogen is made by reforming any hydrocarbon fuel except for coal. Gray hydrogen has been widely used in crude oil refining since the 1950’s – primarily for desulfurization of oil products and hydrocracking heavier feedstocks to make more valuable light products. Since that time, hydrogen use in refineries has become one of the primary tools to meeting environmental regulations on sulfur limits from refined hydrocarbon fuels.
“Blue Hydrogen”:
Blue hydrogen is made from the same fuel source as “Gray”, except that is also integrates Carbon Capture and Storage (CCS) to minimize the carbon emissions associated with the hydrogen production.
“Turquoise Hydrogen”:
Turquoise hydrogen is hydrogen that is created from thermal splitting of methane using methane pyrolysis which generates hydrogen molecules (H2) and solid carbon. This technology has been studied for several years, but the issues with catalyst deactivation by carbon fines have been difficult to solve. In addition, for each tonne of H2 produced there are 3 tonnes of solid carbon to deal with.
“Black or Brown Hydrogen”:
Black or brown hydrogen is hydrogen that is produced from gasification of bituminous (black) or lignite (brown) coal without any carbon capture from the off-gas. The technology utilized for “black or brown” hydrogen is partial oxidation which is discussed under the “gray” hydrogen category in the next section.
“Purple Hydrogen”:
Purple hydrogen uses the heat and power to split water by a combined chemical / thermal electrolysis utilizing nuclear power generation. As of the writing of this tip of the month, it is believed that there have not been any purple or pink hydrogen production facilities commercially applied.
“Pink Hydrogen”:
Pink hydrogen uses nuclear power for direct water electrolysis. This technology would be readily available using current electrolysis options, but there have not been any commercial large scale hydrogen production facilities using this line-up where the nuclear power generation plant has been utilities to solely produce hydrogen to date.
“Red Hydrogen”:
Red hydrogen is produced using a new type of nuclear reactor called a “High Temperature Gas-Cooled Reactor” (HTGR)10. This type of reactor utilizes helium as a coolant, rather than water. As a result, helium can be heated to much greater temperatures (over 1800 oF) as compared to water cooled reactors (600 oF max). The heat created by the HTGR process can be used as the primary heat source for steam methane reforming, thus eliminating hydrocarbon combustion as the heat source for hydrogen production (note the feed source would still be methane).
Hydrogen Colors – Options for Production?
“Green” Hydrogen Production Options:
Two types of electrolyzers are commercially available: alkaline and solid polymer electrolyte (PEM). Both types require a gas-impermeable separator between the cathode and anode to prevent the product gases (H2 and O2) from mixing. The alkaline water electrolyzer is a mature technology. By 1902 there were more than 400 industrial alkaline electrolyzers that were primarily used for ammonia production for the fertilizer industry. These were based on low-cost hydroelectricity. The alkaline process utilizes concentrated lye (KOH) solution as the electrolyte. For “green” hydrogen production, either wind or solar would provide the power required for the electrolyzers.
One of the challenges for this application is the variable load and intermittency of renewable power sources. The conventional Alkaline electrolyzer exhibits poor load following capacity because under partial load conditions the gas impurities increase, which can cause safety shutdowns when approaching the gas flammability limits. Another challenge is the requirement for startup and shutdown cycles due to the intermittency of the power generation. This will lower the expected system lifetime due to electrode degradation.11
Solid polymer eletrolyte (PEM) systems electrolyze water in a cell utilizing a Proton Exchange Membrane. The membrane is permeable to protons, but not to gasses such as hydrogen or oxygen. The membrane acts the separator that keeps the generated hydrogen and oxygen from mixing. The oxygen is evolved on the anode side, and the hydrogen is produced on the cathode side of the membrane unit. The PEM system requires precious metal electrodes (Pt, Ir, Ru). The PEM electrolyzers have better low-load and ramping capability that Alkaline systems, but are more costly.
The other challenge associated with hydrogen production by electrolysis is the amount of demineralized water that is required. A good average for today’s technology is roughly 10 liters/ kg H2 [1.2 gal/lbm H2] produced. To put this into context a 470 kg/h [1030 lbm/hr] electrolyzer would require 4.7 m3/h [2420 gpm] of high-quality water. This is representative of a 1MWe sized unit. 470 kg/h [1030 lbm/hr] of hydrogen production is equivalent to 5 520 std m3/d [196 840 scf/d]. It should be noted that recent projects are in the range of 10 – 30 MWe, which are still significantly smaller than other production technologies. For any baseload energy application, the energy production capacity and availability should be a serious consideration.
In many locations, the freshwater requirement may prohibit this type of technology being deployed as there is insufficient freshwater availability. An example would be California and the Western U.S. where water is scarce and with the growing populations, there is significant stress on local aquifers and reservoirs as it stands without widely deployed “green” hydrogen production.
Today’s electrolyzer technology is roughly 60 – 70% efficient, thus they require 47.6 – 55.5 kwh/kg H2 LHV. Note that the Electrical Efficiency, is defined as shown below:
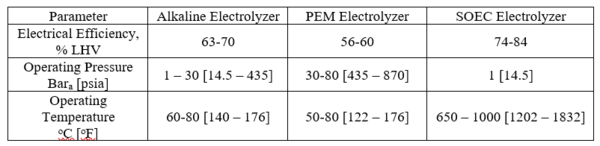
Solid Oxide Electrolysis Cells (SOEC) are being developed and have not been fully commercialized. A comparison of operating conditions and efficiency is provided in Table 112.
Table 112.
Comparison of “Green” Hydrogen Production Technologies
“Gray” Hydrogen Production Options:
Steam Methane Reforming (SMR) is the workhorse in “gray” hydrogen production with roughly 95% of the global hydrogen capacity. According to the IEA13, the global hydrogen demand in 2021 was 94 million tonnes, the majority of which was utilized for refining and industrial purposes. Steam methane reforming uses large, direct-fired heaters to supply heat to the endothermic reaction that occurs between natural gas and steam inside the catalyst filled tubes to create syngas (mixture of CO and H2). The largest units are around 6 million std m3/d [215 MMscfd] of H2 production14. The next step in the process is water gas shift in one or more catalyst filled beds downstream of the fired heater. It should be noted that SMR also requires high quality water, but the water demand is lower than that required from electrolyzer technology.
The main global reactions for Steam Methane reforming are below in Figure 1.:
Figure 1.
Steam Methane Reforming Global Reactions
Autothermal Reforming (ATR) is deployed for large applications, up to 17 million std m3/d [600 MMscfd]. ATR requires a cryogenic air separation unit to supply pure oxygen for the process. The process uses a special burner to partially combust the methane / steam mixture with pure oxygen which supplies most of the heat for the reforming reaction. There is a reforming catalyst bed downstream from the burner, and then the syngas flows on to the water gas shift reactors. The global chemical reactions for ATR are shown below in Figure 2:
Figure 2
ATR main global chemical reactions
Partial Oxidation (POX) is used in large volume applications, like the ATR technology. This process also requires a cryogenic air separation unit to generate pure oxygen. The POX reactor does not use any reforming catalyst. This process can gasify most hydrocarbon feed stocks, from coal to natural gas. The syngas is generated in the POX burner, and then flows to the water gas shift reactors.
Depending upon the produced hydrogen use and purity requirements for the end user, the hydrogen product stream will require a purification step, such as a pressure swing adsorption unit.
“Blue Hydrogen”:
“Blue” hydrogen is essentially “gray” hydrogen production that incorporates CO2 capture in the process unit. The amount of CO2 that can be captured from “gray” hydrogen production depends upon the hydrogen production technology utilized (SMR, ATR or POX), and the location of the CO2 capture process (or processes) deployed within that facility. The maximum amount of CO2 that can be captured ranges from 90 to 95%. “Partial” capture of 60% of produced CO2 from SMRs has been demonstrated with a solvent and with adsorbents – both applied to the shifted syngas.
The challenge associated with adding CO2 capture into current refinery operations is the footprint required for the CO2 capture process equipment. The only commercially applied flue gas capture technology to date has been amine treating which, depending upon the actual volumetric flowrate of gas to be treated, can require very large equipment and process footprint.
The other challenge with “blue” hydrogen is what to do with the recovered CO2. In heavily industrialized areas, there may very likely be a CO2 pipeline available to produce into. If there is no local market, then the issue becomes finding a suitable reservoir that the CO2 can be injected into that is near the project site.
“Turquoise Hydrogen”:
Pyrolysis is the thermal cracking of methane to produce hydrogen and solid carbon, and so it does away with the need for CO2 capture and sequestration. Like power to gas, the technology has existed for decades but due to technical challenges and costs it has never been deployed at commercial scale due to problems with catalyst plugging. New technology developed by scientists at Karlsruhe Institute of Technology and the Institute for Advanced Sustainability Studies (IASS) in Potsdam have developed a new method of pyrolysis that may be promising15.
In short, natural gas is fed into a bubble column reactor filled with liquid tin at more than 1,000 °C [2192oF]. The heat splits the gas into hydrogen and carbon black, or pure carbon in solid form. The heat for the process comes from roughly 10 – 15% of the hydrogen produced15. In December of 2019, KIT announced that it is partnering with Wintershall Dea to further develop this technology to an industrial scale, with the goal of having this work complete within the next 3 years16. Given the current energy crisis in Europe, it remains unknown if this project has made further progress.
Solid carbon, or natural graphite is on the EU’s critical raw materials list and has been since the list’s creation in 2011. Europe imports nearly all its graphite. China is the worlds’ largest supplier of graphite. Primary purposes are for steel making, but it also feeds into high-tech industries such as Li-ion battery production. In addition, graphene is becoming of great interest to many countries. Graphene is a two-dimensional atomic crystal made up of carbon atoms arranged in a hexagonal lattice. It can be thought of as a giant molecule that can be chemically modified, with potential for a wide variety of applications, ranging from electronics to composite materials. The Graphene Flagship project, worth €1bn ($1.12bn) in the EU is the largest research and development initiative to date12.
Although carbon and graphene are valuable products, it should be noted that the global market is still a niche compared to the volume of low-carbon H2 projected for the future net zero energy system.
“Pink, Purple and Red Hydrogen”:
As mentioned previously, at the time of this TOTM writing it is believed that “pink”, “purple” and “red” hydrogen have not actually been pilot tested at a scale that would allow commercial development. HTGR technology, however, has been commercially proven. Two full-scale pebble bed HTGRs are under construction in China with a total 200 – 390 MWe capacity. It will remain to be seen if either one of these are integrated with methane reforming to produce hydrogen as the Japanese group is investigating.
Conclusions:
Hydrogen as a source of clean energy to meet net-zero goals has several technical challenges. The purpose of this TOTM series is to explore these challenges and to hopefully shed some light on what technologies thermodynamically pay out. Currently, only “green” and “gray” hydrogen production is common at commercial applications. Each have their associated benefits and challenges.
“Green Hydrogen”:
Most people can accept that water is a very stable molecule. It has a very high latent heat of vaporization, and a relatively high heat capacity. It takes a significant amount of energy to split a water molecule. This challenge is simply a matter of physics and thermodynamics.
The benefits of the production “green” hydrogen are:
- Potential of utilizing excess energy produced from wind and solar during periods of high production and low demand (duck curve)
- Creates a fuel source that only has the CO2 footprint associated with the production and construction of the wind turbines / and or solar farm and associated maintenance and operation requirements.
The challenges associated with “green” hydrogen production are:
- Intermittency and variable power available for the electrolyzers. Start-up and shut-down on any type of equipment leads to excessive wear, higher maintenance and shortened operational life.
- Intermittency of the hydrogen production because of the issues with the source of the power supply.
- Fresh water requirements for the hydrogen production, and potential water waste stream depending upon technology utilized (water treating requirements and water blowdown streams were not addressed in this TOTM).
- Challenges with storage, transportation and ultimate hydrogen use depending upon the location of the electrolyzers (both water, hydrogen and produced oxygen streams). It should be noted that most wind and solar generators are far removed from areas with industry infrastructure.
- Low hydrogen capacity for baseload use (would require a significant number of electrolyzers and solar or wind power).
- Significant amount of plant footprint (including the wind and solar farm footprints required) and material goods to produce due to the low power density per unit of materials utilized in construction.
“Blue Hydrogen”:
Industrial steam methane reforming is well known technology. The new challenge here would be combining this technology with carbon capture and sequestration.
The benefits of “blue” hydrogen are:
- Very high production capacity for baseload applications
- High energy density
- Well known technology
- Can recovery 90%+ CO2 emissions for sequestration.
- Brownfield units already located in areas of heavy industrial activity (refineries and petrochemical locations).
- The challenges of “blue” hydrogen are:
- Public perception that hydrogen from hydrocarbons is still “dirty” and is “greenwashing”
- Footprint required for the CO2 capture process equipment.
- Not completely “net-zero” emissions
- Requires CO2 infrastructure for take-away capacity (pipelines) and / or development of a new CO2 sequestration injection site with the associated pipeline to transport the CO2. This may or may not be technically feasible. To find a geological formation that is acceptable for CO2 injection and storage can be difficult depending upon the geographic region the facility is at.
- Technical issues with hydrogen storage, transport and distribution depending upon end use.
“Pink, Purple and Red Hydrogen”:
Out of the options to generate hydrogen utilizing nuclear power, “pink” and “purple” would require energy from the nuclear reactor that could otherwise be used for baseload electric power generation. Given that many grids globally are short electrons, it would seem to be a better investment and application to utilize nuclear, including the new small nuclear technology to provide clean reliable energy rather than to use the nuclear energy to generate hydrogen.
“Red” hydrogen is currently being demonstrated by the Japan Atomic Energy Agency and Mitsubishi Heavy Industries, Ltd.17. This technology has the potential of producing large volumes of hydrogen, like the current “gray” hydrogen production options. The benefits of “red” hydrogen is that it integrates waste heat recovery with nuclear power generation, which allows for nuclear power still being provided for baseload electricity generation from the nuclear plant. It is a ‘win-win” but the costs of installing one of these units may be cost prohibitive. In addition, the public perception of the safety risks involved with nuclear reactors may result in permitting challenges that cannot be overcome.
In Closing:
We hope you enjoyed this first white paper on hydrogen colors and how they can be produced. We highlighted some of the benefits and challenges associated with these technologies. For more information on renewable energy options, please refer to our PetroAcademy Low Carbon Energy Options for Power Generation eLearning course. For additional information regarding carbon capture from stationary sources, you may want to consider PF-82 Carbon Capture from Stationary Industrial Sources. On the following page you will find a handy hydrogen summary table that you may like to share with friends and colleagues.
2. “Incentives for Clean Hydrogen Production in the Inflation Reduction Act”, Resources for the Future, Report 22-13, Nov. 2022.
3. Hydrogen Strategy update to the market: July 2022, Department for Business, Energy and Industrial Strategy.
6. https://www.h2-view.com/story/european-commission-plans-to-subsidise-green-hydrogen-production/
8. https://www.nationalgrid.com/stories/energy-explained/what-is-hydrogen
9. https://www.greencars.com/news/is-red-hydrogen-the-breakthrough-technology-weve-been-waiting-for
10. https://www.greencars.com/news/is-red-hydrogen-the-breakthrough-technology-weve-been-waiting-for
11. “Alkaline Water Electrolysis Powered by Renewable Energy: A Review”, J. Bruans, T. Turek, Institute of Chemical and Electrochemical Process Engineering, Clausthal University of Technology, Leibnizstr. 17, 38678 Clausthal-Zellerfeld, Germany. Published: 21 Feb. 2020.
12. IEA, The future of Hydrogen – seizing today’s opportunities, June 2019.
13. https://www.iea.org/reports/global-hydrogen-review-2022/executive-summary
14. IEAGHG, “Current State-of-the-Art Technologies for Hydrogen Production,” in IEAGHG Technical Review 2017-TR3, Reference Data and Supporting Literature Reviews for SMR Based Hydrogen Production with CCS, March 2017.
15. Pyrolysis lifts prospects for hydrogen from natural gas [Brussels Conversation], Natural Gas News, 27 June 2019.
16. KIT and Wintershall Dea collaborating to develop industrial-scale methane pyrolysis for CO2-free production of hydrogen, Green Car Congress, 05 December 2019.
17. https://www.mhi.com/news/220427.html