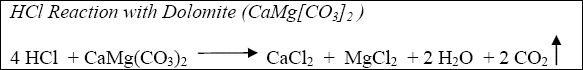The purpose of any fracturing treatment is to improve well productivity or well injectivity, if the well is an injection well. This occurs by placing a high permeability fracture deep into the pay or injection zone. After the fracture has been placed in the formation, it is very important that the fracture conductivity (wkf) be maintained. Today’s Tip of the Month will discuss the strengths and limitations of both acid and proppant fracturing techniques in order to better equip you to make effective well productivity decisions.
PROPPANT FRACTURING
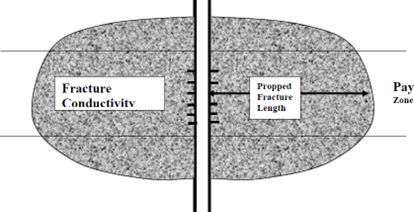
With proppant fracturing, a proppant system capable of withstanding the fracture closure pressure and reservoir environment is used to provide the fracture permeability and to prop the fracture open for sustained production after the fracturing treatment. A schematic of a propped fracture is given in Figure 1.
Figure 1 Schematic of a Propped Vertical Fracture
The characteristics of the propped fracture are dependent upon the placement of inert materials (i.e. fracturing fluid and proppant) into the producing formation. Propped fracturing treatments can be effective in both sandstone and carbonate formations.
ACID FRACTURING
Before the first applications of hydraulic fracturing for well stimulation in the late 1940’s, hydrochloric acid was frequently used to stimulate oil and gas wells producing from carbonate formations. The early acid treatments were matrix treatments with wellbore and perforation cleanout the primary objective. It was also known that, in addition to removing acid-soluble formation damage, the injected acid also reacted with the carbonate formation and improved the near-wellbore permeability.
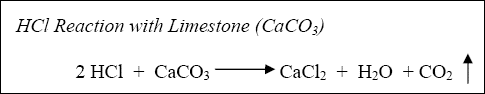
The reaction of hydrochloric acid (HCl) with limestone and dolomite can be described by the following:
Equation 1-1
Equation 1-2
There are two important characteristics of HCl acid reaction with carbonates:
- The acid reaction products are water-soluble
- Considerable amounts of carbon dioxide (CO2) are given off as the acid reacts
Therefore, acid reaction with carbonates is usually considered ‘nondamaging’ and the acid reaction provides a built-in gas assist (CO2) as the acid spends. Gelling the acid with polymers can slow the acid reaction in carbonates and, extend fracture length. Slower reacting organic acids (acetic and/or formic acid) or organic/HCl acid blends are also used in deep wells with high reservoir temperatures (> 200-250o F).
A disadvantage of matrix acidizing treatments is that the acid spends quickly on the formation and the radial penetration of the reactive acid is limited. Although substantial production improvement may be achieved with matrix acidizing treatments in high permeability carbonates or when substantial formation damage is present, the production improvement is not sustained because of the limited extent of formation affected by the acid reaction.
With the early success of proppant fracturing for well stimulation, methods were also developed to inject acid at fracturing pressures to force the acid down the fracture and farther away from the wellbore. Fracture permeability and fracture flow capacity enhancements are made possible with acid fracturing in carbonates by the acid reaction with the formation along the fracture faces.
The acid fracturing process is dependent upon differential etching for the generation of fracture conductivity. Because of their depositional history, most carbonate formations are heterogeneous and consists of gradations from relatively pure limestone (CaCO3) to dolomite (CaMg[CO3]2) to limey dolomite to dolomitic limestone, etc. Anhydrite (CaSO4), which is relatively non-reactive in comparison to acid reaction with the carbonates, is also frequently present in the form of nodules and inclusions.
When acid is pumped down the fracture, the acid removes the reactive carbonate at much faster rates than it does the less reactive portions of the formation. This results in uneven surfaces along the fracture. When the fracture closes, the “pillars and posts” help hold portions of the fracture open. The void spaces in the acid-etched channels create high fracture flow capacity that can provide substantial improvement in production, even when acid fracturing is applied to high deliverability wells.
Properly designed, acid fracturing treatments can be effective even in deep formations that have high fracture closure pressure. With effective acid fracturing, it is critical that the acid-etched fracture stay open so that improved productivity can be sustained. To maintain fracture conductivity, the acidized fracture must be unevenly etched with the unetched portions withstanding the stress of fracture closure.
Because of the rock strength inherent in most limestones and dolomites (with Young’s Modulus ranging from 5 to 10 x 106 psi), stable acid-etched channels can be achieved. However, soft carbonates (chalks, e.g.) that have Young’s Modulus values an order of magnitude lower (i.e. 5 to 10 x 105 psi) may not have sufficient strength to maintain acid-etched fracture conductivity and other means of well stimulation (such as frac packing with proppant) will need to be investigated for such formations.
Another limiting factor that must be considered with acid fracturing is fluid leak-off. A characteristic of acid fracturing that makes it so effective for well stimulation (permeability enhancement along the fracture face due to acid reaction) can also create substantial fracturing fluid loss during an acid fracturing treatment. To generate adequate fracture length, the fluid loss must be controlled to some extent, to allow as deep penetration into the formation as possible.
Techniques have been developed for acid fracturing that help optimize acid-etching in the created fracture and provide longer fracture lengths by improving fluid leak-off control.
An effective acid fracturing treatment will consist of several fluid stages:
o Inert viscous gel pad (usually a stable crosslinked fluid as used for proppant fracturing) to create the fracture system.
o Gelled acid stage to “finger” through the created fracture and to react with the formation along the fracture walls to create channels of high fracture conductivity.
o Inert viscous gel spacer to displace the acid farther into the fracture and to help control fluid leak-off and promote additional fracture growth.
o Gelled acid stage to further react along the acid-etched channels in the created fracture system.
o Inert viscous gel displacement to push the gelled acid stage through the created fracture.
o Closed fracture acidizing stage – pumped with the created fracture closed in order to enhance the near-wellbore acid-etched fracture permeability for sustained productivity improvement.
o Flush – displaces the remainder of the Closed Fracture Acid from the wellbore into the formation.
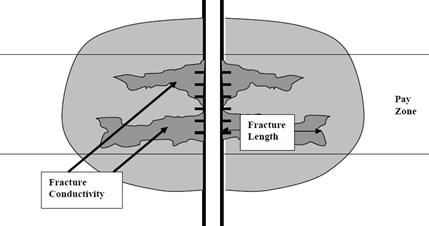
A schematic of an effective acid-fracturing system is shown in Figure 4. The fracture is created by the inert viscous gel and is etched with the stages of stable gelled acid.
Figure 4 Schematic of an Acid-Etched Fracture
Acid Fracturing Fluid Systems
The selection of the fluid system for acid fracturing is critical to the success of the treatment. Therefore, it is important to be acquainted with some of the fluids currently used in acid fracturing treatments:
• Inert viscous gel
• Gelled acids
• Emulsified acid
Types and Strengths of Acids for Acid Fracturing
Hydrochloric acid (at strengths of 15%, 20%, and 28%) has been used successfully for acid fracturing. Although the higher strength acids may require more corrosion inhibitor, their dissolving power is greater (removes more carbonate per gallon of acid) and it is believed the extended spending of the acid (e.g. from 28% to 25%, 20%, 15%, and so on) as the acid is pumped allows longer acid-etched fracture lengths to be generated. However, higher strength acids may cause “sludging” (formation of a viscous oil residue) when the acid mixes with certain crude oils in the formation.
In high temperature applications (> 250o F), organic acid blends (such as acetic acid/formic acid) can have dissolution capacity similar to 15% HCl. The organic acids react effectively at elevated temperatures and are not as corrosive as HCl at those temperatures. Because concentrated organic acids can cause insoluble acetate and formate precipitates in carbonate acidizing, their use is typically limited to 13% Acetic Acid and 10% Formic Acid. Mixtures of 13% Acetic/9% Formic Acid have proven effective for use in acid fracturing carbonate reservoirs exceeding 350o F.
Because acids are so reactive, it is important that each acid blend used for acid fracturing have all the necessary chemical additives to ensure the acid reacts effectively with the producing formation. All acids pumped should contain the corrosion inhibitors necessary to protect tubulars while pumping acid-based fluids and during post-frac well cleanup. Other additives, such as surfactants, mutual solvents, non-emulsifiers, etc. should be added only when proven necessary and effective with your specific rock, acid and reservoir fluids. Diverting agents are chemicals or materials commonly used during acid fracturing to create a temporary blocking effect in perforated intervals already stimulated, or in higher permeability zones, and ensure uniform injection over the area to be treated. All diverting agents are safely cleaned after a stimulation treatment and, many of the most recent products in the market self-dissolve after a certain period of time.
The service company should also verify compatibility of the selected acid fracturing system with formation samples to help ensure that the acid fracturing treatment provides the desired results.
WHICH SHOULD I USE?
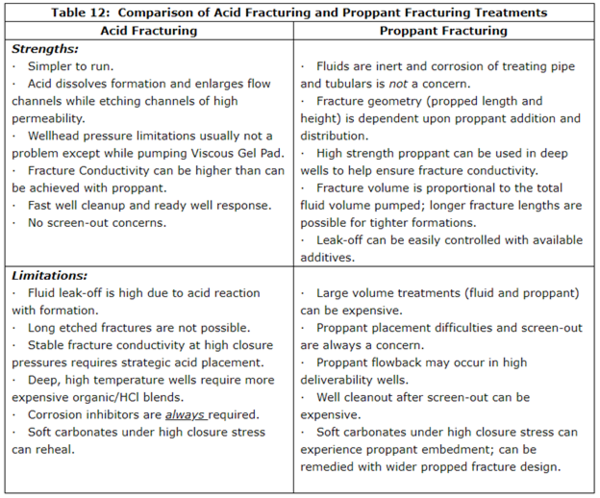
In many producing wells, either acid fracturing or proppant fracturing may be effective in providing improved well productivity. However, each process has its strengths and limitations that should be considered as the fracturing treatment strategy is being developed. A summary of the Strengths and Limitations of Acid Fracturing and Proppant Fracturing is given in Table 12.
The following criteria are also useful in selecting acid fracturing stimulation for a given well:
- Many wells that are successfully stimulated with acid fracturing have been previously stimulated with one or more matrix acidizing treatments. Usually wells that respond well to matrix acidizing will provide better sustained response to acid fracturing. This is logical since more acid (at fracturing rates and pressures) is pumped with the acid fracturing treatment. Careful consideration must be given to previously stimulated wells with matrix acid treatments because, acid, even when pumped at fracturing conditions, may spend much faster and not achieve the desired fracture length after coming in contact with the previously acidized high permeability and conductivity near wellbore area.
- Acid fracturing has been very successful in removing “true” damage (skin) and in reducing non-Darcy flow effects in gas wells producing at high rates. In such wells, usually producing from moderate permeability reservoirs, acid greatly increases the near wellbore permeability and the linear flow through the acid-etched fracture minimizes backpressure effects caused by turbulence.
- Acid fracturing is effective in removing and/or bypassing the effects of severe mud losses that may occur in naturally fractured carbonates during drilling operations. The acid fracturing treatment helps create a primary fracture and also helps restore permeability from the natural fractures that are plugged with mud solids.
- Acid fracturing can be beneficial in stimulating gas wells that produce gas with a high CGR (condensate-to-gas) ratio. Initially the fracture increases productivity and allows the well to flow with higher bottomhole pressures (less drawdown toward the dew point). As the reservoir depletes and pressures decline below the dew point, high permeability acid-etched fractures have proven beneficial in allowing the condensate to ‘drop’ out in the fracture and then to be lifted to the surface with the gas that is produced.
If you would like to learn more about hydraulic fracturing we recommend enrolling in Hydraulic Fracturing Applications (HFU), Advanced Hydraulic Fracturing (AHF) or one of our other Production and Completions courses!